Health economic evaluations of medical devices
TLV performs health economic evaluations of medical devices that are not included in the pharmaceutical benefits scheme but may be procured by the regional councils. An increased knowledge and transparency about the cost-effectiveness of medical devices at a national level may contribute to a more knowledge-based and equitable use of medical devices throughout Sweden.
TLV has been commissioned by the Swedish government to conduct health economic evaluations of medical devices that are not included in the pharmaceutical benefits scheme but may be procured by the regional councils. The health economic evaluations are intended to support clinical decisions and procurement decisions taken by the regional councils. An increased knowledge and transparency about the cost-effectiveness of medical devices at a national level may contribute to a more knowledge-based and equitable use of medical devices throughout Sweden.
Since 2020, the Medical Technology Product Council (MTP-rådet) within the regional councils' collaboration model for medical technology (Regionernas samverkansmodell för medicinteknik) selects medical devices to be evaluated on a national level and consequently issues national recommendations regarding use of the evaluated medical devices. The health economic evaluations, performed by TLV, are thus part of the national process for managed introduction of medical devices.
 Zoom image
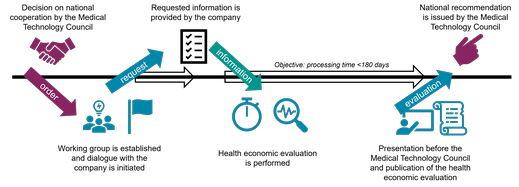
Zoom imageThe illustration shows the process of a Health economic evaluation. Click to zoom in.
A health economic evaluation usually refers to a specific medical device. However, TLV may perform several health economic evaluations in parallel to cover a group of medical devices.
In a health economic evaluation, the costs, and clinical effects upon use of the evaluated medical device are compared to the costs and clinical effects upon use of a relevant comparator with regard to Swedish conditions. For more guidance on the content of a health economic evaluation, please read the TLV´s general advice on economic evaluations (only available in Swedish). Link is in the green box "Related information".
The basis for a health economic evaluation, performed by TLV, is mainly information submitted by the company that provides the medical device to be evaluated. The company’s submission should contain information about the medical device, how and for which patients it is intended to be used. The companies' dossier should also contain a health economic analysis, where costs and revenues are related to clinical effects in relation to a relevant comparator. The clinical effect can be supported by published literature, but also yet unpublished data and/or studies. TLV may also, if necessary, add data to the investigation from other sources, e.g., evaluations from other countries and/or scientific studies.
The time needed for a health economic evaluation, counted from the order to the delivery of a report to the Medical Technology Product Council (MTP-rådet) varies and is e.g. dependent on the time that the concerned company needs to submit information to TLV. Preferably, the TLV's processing time, counted from the company’s submission to delivery of a report, should not exceed 180 days.
Based on the health economic evaluation by TLV, the ethical platform for prioritization in healthcare and potentially other relevant sources of information, the Medical Technology Product Council (MTP-rådet) may issue a national recommendation to the regional councils regarding the use of the evaluated medical device.
Page information
- Last updated
- 1 August 2022